- What existing solutions/examples have you implemented or heard about?
- How is the movement of the (usually working) electrode in and out of the electrolyte handled?
- How are automated electrical disconnects and re-connects handled, if at all?
- How do you approach liquid handling for hazardous materials (e.g., KOH) which can also be at high temperatures? (e.g., materials used, safety / robustness / cost)
Here are some references you might find interesting:
Parallel Reaction System: https://pubs.acs.org/doi/10.1021/acsmeasuresciau.3c00022
Homemade System (similar to our OT setup): DOI: 10.1016/j.device.2023.100103
John Gregoire’s Work on HT Electrocatalysis Measurement: This paper includes a system capable of collecting gas products, which the above two systems lack. https://pubs.acs.org/doi/full/10.1021/acsenergylett.1c02762
The ability to handle hazardous materials depends on the materials used to construct the reactor. Most reactors should be capable of this, especially those made with PEEK. However, high-temperature reactions are more challenging, as they often require reactors made from stable, heat-conductive metals like titanium. This can introduce risks such as metal contamination in the reaction and metals are usually electrical conductive. So, we typically use PEEK, although it is unsuitable for high-temperature reactions. Recently, Kelvin and I have been discussing materials that are non-Econductive, thermally conductive, and chemically resistant for reactor construction—Kelvin might have some ideas on this.
I’ve been working with Matthias Gramlich and Bojan Micevski on a 3D printed electrochemical cell for fuel cell catalyst tests. Most of the development history including discussion, images, videos, and links to CAD files are available in the following issues:
- Custom electrochemical cell (v2) - enhancements and automation · Issue #104 · AccelerationConsortium/ac-training-lab · GitHub
- Custom Sample Holder (v3) - design and automation · Issue #124 · AccelerationConsortium/ac-training-lab · GitHub
- Custom electrochemical corrosion cell for electrocatalysis experiments (v1) · Issue #62 · AccelerationConsortium/ac-training-lab · GitHub
I’ll get something more condensed soon, and full docs will move to GitHub - AccelerationConsortium/echem-cell: A custom, 3D printed electrochemical cell for autonomous electrochemistry, targeted at fuel cell electrolysis studies. (as of 2025-04-04 currently just placeholders).
You can see the cell in operation at the back of this picture:
- My exposure to existing solutions for HT electrocatalysis and screening is down to redox.me and IKA. Whilst their solutions are quite decent, I feel that they’re a bit too dear for many labs that might want to experiment with electrochemistry, and this domain isn’t their strength. Investing significant sums (up to US$2k in some instances!) might be a bit much if one’s not sure if the process will work for them!
- The electrodes, in the form of plates, are manually inserted into the electrolyte chamber after the chamber has been filled with the required volume of electrolyte. The process is currently not automated, which is one of the reasons our research team implemented the CNC-based liquid dispenser that can work with organic solvents, allowing for the dispensing of organic electrolytes.
- I’m not aware of any automated solutions for handling the connection and disconnection of electrodes to the potentiostat cables. I’d be keen on any idea that helps us smooth this process!
- The considerations and allowances we must make for handling organic solvents are paramount; all components that may come into contact with the electrolyte should be resistant to the solvent in question. For electrosynthesis, if we were to avoid the dark road of fluoropolymers (reducing our dependency on fluoropolymers is a key goal of our lab, so I’m not inclined to pursue the path of fluoropolymers), we must keep solvent compatibility in mind whilst preparing our components. I would absolutely test the solvent compatibility myself before committing, as I learnt this the hard way!
Neat electrochemical cell design by @dlin and folks as part of a workflow involving “seven robots with automated modules for gas diffusion electrode fabrication and characterization and an automated test cell (ATC) that performs zero-gap CO2 electrolysis”:
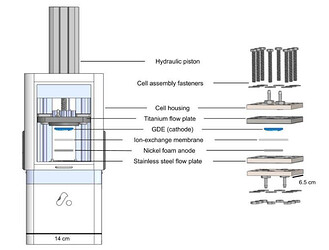
Comparison of the models of the automated test cell (ATC) described herein and a manually assembled electrolyzer. The ATC is structured similarly to a manually assembled electrolyzer using the same stack of flow plates, cathode, anode, and ion-exchange membrane. However, the ATC has no assembly fasteners but instead uses a hydraulic piston to automatically close the cell. The data acquisition and processing, including experimental conditions and the calculation of Faradaic efficiency (FE), is completely automated for the ATC system.
Design of ATC cathode and anode plates. The anode flow plate is made from stainless steel and has a 1 mm deep × 1 mm wide channel that was machined in a serpentine pattern with an active area of 20 mm × 20 mm. The cathode flow channel plate has the same features as the anode but is made from titanium. Around the channels of each plate is an O-ring groove with an embedded Viton O-ring to aid in sealing the cell when pressure is applied. Each flow channel plate is embedded in a 3D-printed, plastic housing which incorporates ports for inlet and outlet fittings, as well as mounting features to the aluminum frame of the ATC.
Photograph of the ATC platform showing individual components.
@dlin feel free to weigh in with other thoughts or lessons learned from the automation side of this.
Thanks for highlighting our team’s work! Karry Ocean was the design lead for this project, so he’ll have more technical info if you contact him.
A major takeaway is that this is a very difficult automation challenge and it took a fairly complex engineering design to solve. As mentioned before, material compatibility is important. It’s also important to know ahead of time what parameters you want to manipulate (flow rates, temperatures, humidity, current density, etc.) and what data you want to measure (FE, voltage, actualized flow rates, pH, etc.). Identifying these early and designing for them makes it much easier down the road as it can be very difficult to add in new functionality later for a module like this.
Handling leaks and flooding is also key. If handled improperly, you can flood and damage the mass spectrometer/GC (we learned this the hard way). I believe there are 2 safety mechanisms in place for catching leaks - one is a liquid detector that detects liquid in the tubing and automatically actuates a diverting valve to divert the stream to waste before reaching sensitive equipment. The other is a liquid catch, in case the first mechanism fails.
I believe there was also significant challenge when it came to data processing and calibration for the mass spectrometer. I wasn’t involved in that process so I don’t know much though.
We implemented a GUI that would also depict in real time what the path of all the gases and liquids were, based on the state of the sensors and valves and the workflow. This was helpful for understanding the system and allowed a user to know what was going on, especially if they ran into issues.
hey @dlin, thanks a lot for sharing details about your very interesting project. I definitely believe, that it was quite challenging to built this setup abut on the other hand it was probably quite a cool project, too.
If I understand the paper that Sterling linked correctly, the exchange of electrodes in the test cell was done manually. Did you also consider implementing an automated workflow here? If so, could you share details about how such an approach could look like?
I guess, you controlled the potentiostat via a (python) API? Can you share some details about challenges you might have had with this?
One thing that turned out to be a way of handling highly aggressive (electrolyte) solutions in setups which would be attacked by these substances turned out to apply a coating of chemically resistant epoxy. So far we have made good experience with epoxy from the novolac family.
Hi @mgramlich, great questions!
Correct, the exchange of electrodes in the cell was done manually. One of the things we’ve been working on lately has been automating this process, but this is actually a far more challenging process than it seems for a number of reasons:
- Our GDEs are mounted to a microscope slide during the fabrication and characterization process, but a 20mm x 20mm square needs to be removed from it prior to loading into the test cell.
- Once sprayed, we want to avoid contacting the sprayed surface of the GDE
- The GDE needs to be oriented sprayed-surface down within the test cell
- The Sustainion membrane is stored in KOH solution and curls aggressively as it dries. It is also very susceptible to being punctured if handled improperly.
These challenges result in a substantial automation project in and of itself, which we unfortunately have not had the funds or bandwidth to focus on.
As a proof of concept, we have used a robot and custom automated equipment to punch out the GDE, pick it up with a Bernoulli gripper, and load it into a mock test cell. We’ve also done some work into material preparation techniques and frames to make the floppy & curly membranes handle-able by a robot. However, this hasn’t been tested extensively or integrated yet.
As for the potentiostat control, from what I know our Python code sends a script that is interpreted and run by the Keithley potentiostat. I wasn’t too involved in that development though, but I recall my colleagues having not much issue with the Keithley (but lots of problems with other fancier potentiostats we were intending to use, like an Ivium potentiostat).
hey @dlin, thank you very much for the feedback. I absolutely agree, that automating the exchange of this kind of samples is very challenging and quite a project to tackle. Especially, when aggressive solvents like KOH come into play, I imagine that one needs to be sure the system is failsave.
Can you share roobot you are using for the proof of concept experiments and if you are satisfied with it?
Also, if you could get some details about the problems your colleagues had with the ivium potentiostat, that would be very interesting for me, as I would only have access to an ivium atm. So far I only read their documentation and talked to the support who assured me, that the python API would be functional.
I asked my colleagues why we didn’t use the Ivium and it turns out there was a software bug on the Ivium side that they hadn’t yet resolved at the time (the company was aware of the issue but had no timeline for the fix). The bug made it incompatible with our system so we dropped it in favour of the Keithley. They very well could have fixed it by now, and/or it may not be an issue with your system as well.
The robot we’re using for proof of concept was the UFactory Arm Lite 6. We actually didn’t select this robot for this application; if anything we used this application as a proof-of-concept to test the performance of the robot arm (it was so cheap that we wanted to see how good it was). We’re not overly impressed with it, but it was definitely “good enough” for the movements we were testing. Given the right application it’s definitely a great option for the price, but not something we’d put on a highly precise or critical operation.
Very interesting, thanks for the update on not using Ivium. I very much hope, that they have fixed the pyvium library as for now the moment the only potentiostat in our labs having a python api at all is the Ivium. Once I start playing around with it, I´ll report my experience with here.
Also thanks for the information about the robot you used. May I ask which robot you would use today if you could choose completely freely?
Following is another one worth considering, which the AI & automation lab has several of (and overall good feedback):
Overall I think folks at AC have been satisfied with X-ARM 6 (the full, non-lite version) when they’re more budget constrained, but still want something with reasonable accuracy / good software ecosystem / “cobot” safety features.